The recent rise in recalls of medications found to have N-nitrosodimethylamine (NDMA) may seem overwhelming, however, with the right tools and information, you can stay on track and take control of your medications.
What is NDMA?

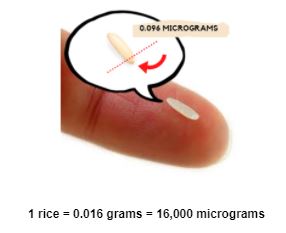
NDMA, a nitrosamine, is a chemical impurity that can be found in drinking water, foods (such as fish, cured meats, and cheese), vegetables, tobacco, and even medications. These are minor sources of exposure, with NDMA levels below 0.0021 micrograms per liter in water, 0.00037 micrograms per gram in meat, and 0.000075 micrograms per gram in vegetables for example. NDMA can be found in medications for many reasons, including as a result of the way it is manufactured, stored, or packaged. Taking small amounts of NDMA over a long period of time is not dangerous; however, taking large amounts can increase your risk for cancer and liver damage. The United States Food and Drug Administration (FDA) sets a daily intake limit of 0.096 micrograms (96 nanograms) for nitrosamines in medications, as this is considered a safe amount for lifetime exposure. Medications are recalled if they have nitrosamine amounts over the FDA limit.

What is a Recall?
When an unexpected problem occurs after a product is widely distributed, it can prompt a recall. A recall is when a product is removed from the market or taken back to be corrected. A company might discover a problem in one of its products and voluntarily recall it, or the FDA becomes aware, alerts the public, and requests the company to recall its defective product.
Pharmaceutical manufacturers will inform pharmacies about which products are being recalled so that they may be removed from the pharmacy’s inventory. Pharmacies then review their records to identify and contact patients who received the recalled product. Pharmacists and providers work together to find an alternative medication for the patients. The recent increase in medication recalls may be attributed to advanced technology and better testing methods, allowing even miniscule amounts of NDMA in products to be more easily recognized.
This issue was first discovered in 2019, when the FDA was informed of the presence of NDMA in international metformin products. The FDA then began testing the metformin products in the United States, to identify and remove medications with high nitrosamine levels from the market and ensure public safety. At this time, unacceptable levels of NDMA have only been found in CERTAIN extended release (ER), no immediate release (IR), metformin products. Some metformin products have shown no detectable levels of NDMA, while others have shown levels ranging from 0.005 to 0.19 micrograms. Click here, to find an up-to-date list of companies currently impacted by the metformin recall.
Of course, metformin is not the first medication to be pulled from the market due to unacceptable NDMA levels.
Other medications that have been recalled include:
Zantac (ranitidine) ⇒ Used to treat:
heartburn & acid reflux
Valsartan, Losartan, Irbesartan ⇒ Used to:
lower blood pressure
It is important to note that, just like valsartan, losartan, and irbesartan, metformin has a nationwide recall – meaning that the recalled products may be found and sold internationally. Not all medications outside the United States are regulated by the FDA. Recalled medications should be avoided, even if available overseas. In contrast to metformin, NDMA was found in all  tested samples of Zantac (ranitidine), with levels ranging from 0.01 to 0.86 micrograms, leading to a global recall. The FDA also found that NDMA levels in ranitidine increased with time and temperature, and requested all products to be withdrawn from the U.S. market. To date, NDMA has not been found in other products that are used for the same treatment, such as Pepcid (famotidine), Nexium (lansoprazole), or Prilosec (omeprazole).
tested samples of Zantac (ranitidine), with levels ranging from 0.01 to 0.86 micrograms, leading to a global recall. The FDA also found that NDMA levels in ranitidine increased with time and temperature, and requested all products to be withdrawn from the U.S. market. To date, NDMA has not been found in other products that are used for the same treatment, such as Pepcid (famotidine), Nexium (lansoprazole), or Prilosec (omeprazole).
How Should You Respond?
Recalled products are not always dangerous. Many times, recalls can be precautionary, meaning that the FDA is proactively working to guarantee your medications are both safe and effective. Remember, not all metformin products were found to have NDMA. Safe and alternative options are available, and your doctor can help you decide what options are best for you.
Do not stop taking your metformin. If you have Type 2 Diabetes, it is important to continue taking your metformin as your doctor prescribed, to keep your blood sugar controlled and to avoid any complications. Pharmacies, like CVS Health and Express Scripts, are mailing out letters and notifying their patients who received the recalled metformin. They are working with patients and/or their prescribers to replace their metformin or obtain a new prescription. Depending on individual circumstances, patients may have the following options:
- Switch to a different manufacturer
- Switch to a different formulation
(such as immediate release instead of extended release) - Switch to a different medication

Talk to your doctor to discuss your options and to check if the medication you have at home is affected by the recall. If your doctor recommends changing your medication, continue taking your metformin until your doctor or pharmacist provides you with the replacement. Be sure to ask your pharmacist how to properly dispose of your metformin.
Adverse reactions or quality problems related to metformin can be reported to FDA’s MedWatch Adverse Reporting program online (http://www.fda.gov/MedWatch/report.htm), by mail (pre-addressed Form FDA 3500 available at: http://www.fda.gov/MedWatch/getforms.htm), or by fax (1-800-FDA-0178). If you have any questions or concerns about your medications, contact your doctor or your pharmacist.
Lastly, stay updated. Visit the FDA website here to keep track of all medication recalls.

For More Information:
https://youtu.be/UF18Yykk3E4
https://youtu.be/TKUIlfdPam0
Drug Take Back Locations
Medicine Flush List
References:
Visit the Website
Nitrosamine Impurities
NDMA Metformin
Nitrosamine Impurity Findings Certain Metformin
NDMA Metformin Announcements
Lab Tests for Metformin
NDMA Metformin Updates
Download the PDF
Author:
Aarezo Riaz – is a PharmD candidate at VCU School of Pharmacy. She is driven to promote patient education, empowerment, and advocacy. She enjoys speaking with patients, and individualizes every interaction to ensure her patients feel valued and understood. Her interests include academia, ambulatory care, and public health. You can connect with Aarezo at linkedin.com/in/aarezo-riaz.